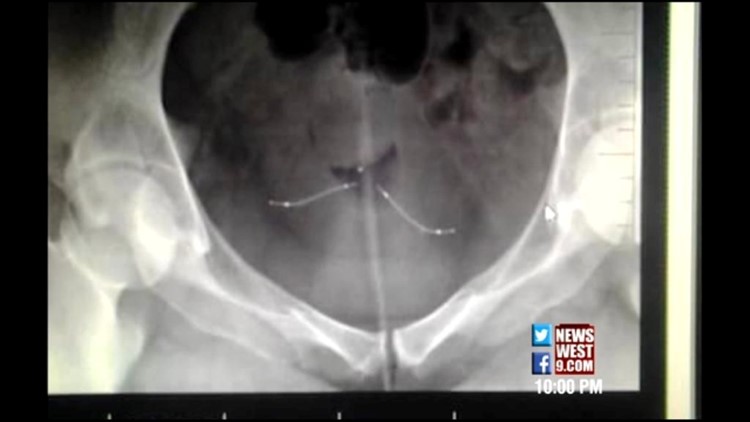
The U.S. Food and Drug Administration is re-examining the safety of Essure, a contraceptive device linked to at least five deaths and complications in thousands of women.
The FDA announced Wednesday its Obstetrics and Gynecology Devices Panel will hold a public meeting on September 24 in Washington, D.C. to discuss the "safety and effectiveness" of the controversial birth control method. Members of the public are invited to make recommendations during the meeting.
The FDA also added new information Wednesday for doctors performing the Essure procedure, including a warning about nickel allergies.
"The FDA takes reports of safety concerns seriously," the agency said in a statement. "Over the past several years, the [FDA] met with patients and patient advocates to better understand patient issues and experiences after Essure placement."
At least 5,000 reports of serious complications associated with Essure have been filed with the FDA since it was introduced in 2002. Multiple deaths and late-term miscarriages have also been linked to the Bayer-manufactured device.
However, the pharmaceutical company has publicly stated that "death is not a known failure mode of Essure" and continues to market it as a permanent, non-hormonal birth control option that requires "no downtime to recover" and is associated with "rare reports of chronic pelvic pain." Essure is currently offered in at least 23 countries and has been implanted in approximately 750,000 women worldwide, according to Bayer officials. A full statement from the company can be found below.
The device consists of two metal coils placed in the Fallopian tubes by a catheter passed through the cervix and uterus. After about three months, sufficient tissue growth in and around the coils permanently blocks the Fallopian tubes, preventing conception.
However, women have reported serious side effects, including perforation of the uterus and other organs, fragmentation of coils, autoimmune symptoms mimicking arthritis and fibromyalgia, unexpected pregnancies and miscarriages, unmanageable pain and severe menstrual bleeding.
Permian Basin women who faced serious Essure complications have joined the fight to pull the device off the market.
"My whole uterus is pretty much just scar tissue now," said Kristen Jones, 29, a mother of three boys. "I've got a doctor who's willing to do my hysterectomy. Unfortunately, that's the only way for me to have mine removed because [one coil has broken into fragments] and [the other coil is] more than halfway into my uterus."
She told NewsWest 9 the metal device could "poke through [her] organs any day now."
According to Jones, her doctor failed to inform her of the full scope of side effects before inserting the coils.
"All they told me was, 'It'll take ten minutes, you don't need anesthesia and there's no downtime,'" she said. "Even putting it in was painful for me. [All my other symptoms] started three days later."
41-year-old Elvira Lopez told NewsWest 9 a similar "horror story." She, like Jones, was not informed of possible complications and opted for Essure because it seemed like a "cheaper, easier" alternative to tubal ligation.
Pain, bleeding, tissue scarring, autoimmune disorders - among other symptoms she believes was a direct result of Essure - forced her to undergo a hysterectomy two months ago.
"I got diagnosed with [everything] from arthritis to fibromyalgia," said Lopez. "Finally, I found a doctor who believed me when I said it was the Essure. Everybody else had looked at me like I was crazy. I just cried when he said he believed me."
She immediately felt relief following her hysterectomy, she said, and is slowly regaining her health.
"My little boy was 3 when I got [Essure]," she told NewsWest 9. "He's now 7. I feel like I missed part of his childhood."
More than 13,000 people have signed a petition backed by consumer advocate Erin Brockovich to take Essure off the market. A Facebook group called "Essure Problems" now has more than 18,000 members; local "E-Sisters" estimated at least 700 live in Texas.
"Ideally, it would've been amazing for it to be called off the market," Jones said in response to the FDA's announcement.
She said holding a public hearing to reexamine Essure "is still a huge step forward" and plans to attend the meeting in September.
"I want [the FDA panel] to know what I had to suffer through," said Jones. "If this [testimony was coming from] their sister or wife, would they be... behind us, fighting?"
Bayer HealthCare representatives sent the following statement to NewsWest 9:
"Bayer looks forward to an open and transparent discussion regarding Essure at the meeting of the Obstetrics and Gynecology Devices Panel on September 24, 2015. Bayer has been in regular communication with the FDA about the risk-benefit profile of Essure and the informational needs of both healthcare providers and patients.
"Patient safety is Bayer's top priority. Given there has been a great deal of interest in the safety of Essure among some patients, we welcome this open dialogue with healthcare providers, patients, researchers, representatives from professional societies, and other members of the public to review and discuss available data regarding the benefits and risks associated with Essure.